Serious Pelvic Infections and Toxic Shock Syndromes
Authors
INTRODUCTION
The obstetrician/gynecologist commonly faces the challenge of diagnosing clinical infection and initiating antimicrobial treatment. Many of the obstetric and gynecologic infections we treat are potentially life threatening. Although such infections are uncommon, early recognition of sepsis with prompt medical therapy to include appropriate antibiotics and judicious surgical intervention can lead to the successful treatment of these potentially life-threatening infections in most circumstances.
This chapter does not address the routine postpartum or postoperative infections that respond promptly to antimicrobial treatment. Instead, the chapter focuses on a common reason for treatment failure in the treatment of routine postpartum and postoperative infections: pelvic abscess formation. It also covers the diagnosis and treatment of several toxic shock syndromes, necrotizing fasciitis, and finally septic shock.
PELVIC ABSCESS
An abscess is a circumscribed collection of pus formed by liquefaction necrosis within tissue. Fibrous tissue is deposited around these pus collections if they are not drained. This tends to isolate the purulent collection further, serving to localize microbial enzymes or toxins injurious to the host, thus making it more difficult for antimicrobial agents to penetrate the capsule and sterilize the contents. In addition, local exhaustion of complement and enzymatic degradation of immunoglobulins occur, favoring the persistence of the bacterial infection.1
Abscesses complicate both hysterectomy and cesarean delivery as well as forming as the result of pelvic inflammatory disease (PID). The clinical diagnosis is generally based on the findings of fever and a palpable adnexal or pelvic mass. This mass may be distinctly palpable and fluctuant or less distinct and characterized by a “fullness” noted during bimanual pelvic examination. A pelvic abscess can develop despite appropriate antibiotic treatment for postoperative soft-tissue infection or PID. Abscess formation is a common reason for a patient failing to respond to an initial course of antibiotic therapy. Clinical manifestations include a persistent spiking temperature along with a leukocytosis and elevated erythrocyte sedimentation rate or C-reactive protein.
Imaging techniques are helpful in characterizing the size of the abscess and in determining whether the collection of purulent material is unilocular or multilocular. Patients with a true localized unilocular, purulent collection will be more likely to require surgical drainage. An image showing a multiloculated mass may reflect inflamed pelvic tissues and bowel often adhere to one another with associated small pus collections. These masses have been referred to as tubo-ovarian complexes. Patients with tubo-ovarian complexes are more likely to respond to antibiotic treatment alone.
Ultrasound (US) is the first method of choice to evaluate a pelvic mass thought to be an abscess. It easily differentiates between fluid-containing and solid lesions. Pus collections have fine internal echoes of differing sizes. Transvaginal sonography can augment the findings noted during abdominal scanning.
Computed tomography (CT) or magnetic resonance imaging (MRI) are cross-sectional imaging methods that generate information similar to that noted on transverse sections on ultrasound. CT examination is optimally performed after the patient is administered oral contrast to opacify the bowel loops and intravenous contrast to identify vascularity and to opacify the urinary tract. Like ultrasound, CT or MRI can locate inflammatory masses and abnormal fluid collections, but is used more often than US to search for a suspected abscess in a postoperative patient in which an US examination is limited by open surgical wounds and abundant bowel gas. An abscess has a low-density center if liquefaction has occurred. A thick wall may be demonstrated, depending on the age of the abscess.2
The microbiology of pelvic abscesses is predominately anaerobic. The intra-abdominal abscess rat model of Weinstein and colleagues3 is an excellent description of the phases of pathogenesis of mixed aerobic–anaerobic infections of the abdomen and pelvis:
Phase I: Initial stage of peritonitis, sepsis, and an associated high mortality rate of nearly 40% appeared to be due to facultative Gram-negative bacteria, particularly Escherichia coli.
Phase II: Abscesses developed in the surviving rats during the secondary phase of infection due to anaerobic bacteria, particularly Bacteroides fragilis.
A similar biphasic disease process can occur in PID, pelvic cellulitis, and endomyometritis which are analogous to the initial phase of peritonitis in this model.3 These infections can progress to phase II characterized by pelvic abscess formation.4
The traditional approach to the treatment of mixed anaerobic–aerobic soft-tissue pelvic infections sheds additional light on the pathophysiology of abscess formation and on the importance of using antimicrobials with activity against penicillin-resistant anaerobes as part of initial therapy.5 Initial treatment with penicillin and gentamicin or with ampicillin alone has a relatively high failure rate. Most initially resistant infections respond to the addition of an antibiotic with extended anaerobic activity (e.g., clindamycin, metronidazole). Patients who do not improve with broad-spectrum antimicrobial therapy should be evaluated for the presence of an abscess, septic pelvic thrombophlebitis, or drug fever.
Broad-spectrum antibiotics are given as the initial treatment for patients with a diagnosis of pelvic abscess. The gold standard of antimicrobial regimens for the treatment of pelvic abscess is combination therapy with either clindamycin or metronidazole in conjunction with an aminoglycoside, third-generation cephalosporin, or aztreonam. Other agents with therapeutic utility include single-agent treatment with an extended-spectrum cephalosporin (e.g., cefoxitin, cefotetan, cefotaxime, ceftizoxime), an extended-spectrum penicillin (e.g., mezlocillin, piperacillin), carbapenems (imipenem, meropenem, ertapenem), and β-lactamase inhibitors plus a β-lactam (e.g., ampicillin/sulbactam, ticarcillin/clavulanate, piperacillin/tazobactam; see ahead to Table 2).
When antimicrobial treatment is started, a decision on the need for surgical intervention is required. Pelvic abscesses will commonly respond to antimicrobial treatment alone. This is particularly true in patients with pelvic inflammatory disease that is complicated by a tubo-ovarian abscess. Treatment with broad-spectrum antimicrobials results in a satisfactory response to therapy without the need for surgery in 75% of cases.6 Most abscesses complicating posthysterectomy infections are cuff abscesses and can be easily drained by dilating the vaginal cuff. Patients with postoperative adnexal abscesses (tubo-ovarian abscesses) are less likely to respond to antibiotic treatment alone, but trial use of antibiotic therapy is warranted before surgical drainage. An adnexal abscess that fails to respond to antibiotics should be drained.7
Techniques recommended for the drainage of pelvic abscesses have evolved. In the past surgeons preferred laparotomy and drainage, with or without extirpation of the infected tissues, as the treatment of choice. Now, laparoscopic approaches are now being used to drain or excise infected tissue. However, percutaneous drainage guided by CT or US is now the procedure of choice for drainage of a pelvic abscess with success rates of 80–90%.8, 9 The choice of options is based on the skills and facilities available at each hospital.
A ruptured pelvic abscess remains a surgical emergency. Such patients generally present with persistent fever, increasing leukocytosis, and a rigid abdomen. Immediate surgery after the initiation of antimicrobial therapy and fluid resuscitation is necessary. Standard treatment consists of drainage of the pelvic abscess and copious irrigation of the abdominal cavity. In some cases removal of the adnexa is necessary for cure and should be considered, particularly in women who have completed their families or in postmenopausal women.
SEPTIC PELVIC THROMBOPHLEBITIS
Septic pelvic thrombophlebitis (SPT) is a rare but potentially serious complication of postpartum infections. The incidence of this disease ranges from 0.03% to 0.18% of obstetric procedures, including cesarean delivery. The disease occurs even less frequently after gynecologic surgical infections.
The pathophysiology of SPT is outlined by Virchow's triad:
Venous stasis
Injury to the vascular epithelium
A hypercoagulable state
These factors explain why SPT is almost always a puerperal event that is most commonly diagnosed after postcesarean endomyometritis. Pregnancy predisposes the patient to a hypercoagulable state. Infection is believed to be the cause of injury to the vascular epithelium. After delivery, pelvic veins collapse and stasis occurs. The ovarian veins, particularly on the right, tend to be involved. Approximatly 40% of the time, the smaller uterine pelvic veins are thrombosed. This small vessel thrombosis may be overlooked by CT or MR scanning. The diagnosis of SPT can be made when the patient has persistent fever despite 96 hours of antimicrobial treatment for postpartum endomyometritis and imaging confirms pelvic vein thrombosis. The patient appears well except during the febrile spikes and usually has a normal physical examination. The differential diagnosis includes drug fever.10, 11, 12
The diagnosis of SPT is distinctly different from the diagnosis of the patient presenting with an acute ovarian vein thrombosis (OVT). Patients with OVT are acutely ill and in pain. They present with localizing tenderness and a midquadrant mass. A computed tomographic scan confirms the presence of a clotted ovarian vein (Table 1).
Table 1. Pelvic vein thrombophlebitis10
Acute ovarian vein thrombosis | Septic pelvic thrombophlebitis |
Onset 2–4 days after surgery | Onset 4–8 days after surgery |
Acute onset | Slower evolution |
Acutely ill | Appears well |
Localized pain | Painless |
Midquadrant mass | No mass |
CT scan usually abnormal | CT scan can be normal |
CT scan = computed tomographic scan.
The use of anticoagulation can be used as a diagnostic test for SPT as the patient's response should be prompt, becoming afebrile within 24 hours. The treatment of SPT includes the continuation of broad-spectrum antibiotic therapy. The use of an antibiotic (e.g., clindamycin or metronidazole) with a spectrum of activity against anaerobic bacteria is important because some of these microorganisms have been shown to produce a heparinase and may lead to persistent or progressive disease. The patient should be therapeutically anticoagulated, although response occurs in patients treated with heparin despite a normal coagulation profile. Treatment need only be continued until the patient is afebrile for 48 hours. No long-term or outpatient therapy is needed. The cause of the thrombosis is related to the infection and pregnancy (both are nonrecurring risk factors). Anticoagulated patients with pulmonary embolism should be considered candidates for inferior vena cava ligation or for placement of a Greenfield filter. Hysterectomy is not indicated in these patients.
In those patients not responding to heparin, a diagnosis of refractory postpartum fever can be made. Anticoagulation may be stopped. Management of these patients is continued antibiotic therapy and observation if pelvic abscess has been ruled out. Given that most of these patients feel well, outpatient treatment with close followup is acceptable. The natural history of disease in these patients is gradual resolution of fever, averaging 12 days.
NECROTIZING FASCIITIS
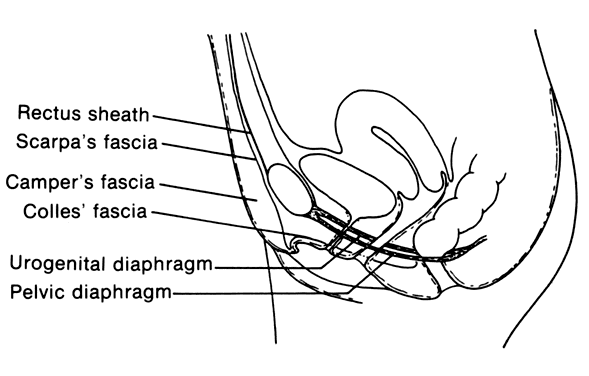
Necrotizing fasciitis is a serious infection of the superficial fascia and is associated with extensive necrosis of the superficial fascia and subcutaneous fat.13 The most common sites affected in the obstetric and gynecologic patient are the vulva and the anterior abdominal wall. This process starts with a simple infection of the subcutaneous tissue often associated with an abrasion or furuncle or a surgical wound. The inflammatory process then extends along superficial fascial planes (Fig. 1).14 Thrombosis of small vessels occurs, devitalizing the subcutaneous tissue and resulting in the destruction of superficial nerves. The anatomic realities of this disease explain its spread. The superficial fascia of the vulva (Camper's fascia) is contiguous with the same fascia on the anterior abdominal wall and inner thighs. In addition, this fascia comprises most of the labia majora. The deeper fascia of the vulva (Colles' fascia) is contiguous with Scarpa's fascia of the anterior abdominal wall. These planes allow the infectious process to spread from the vulva to the anterior abdominal wall and into the inner thighs.
|
Patients with this disorder invariably have an underlying disease that impairs host immunity. Diabetes mellitus is the most common predisposing disease, but women with atherosclerosis or those who are on steroid therapy are also at risk. Rarely, necrotizing fasciitis develops in postpartum patients who have had episiotomies. Necrotizing fasciitis in the retropsoas and subgluteal spaces complicating pudendal anesthesia also has been reported.
The bacterial pathogenesis of necrotizing fasciitis is polymicrobial. Aerobic and anaerobic bacteria, especially streptococci, E. coli, Clostridum sp., and Bacteroides sp., found in the genital tract can manufacture proteases that break down collagen and elastin and allow the infection to spread along tissue planes. In addition, Streptococcus pyogenes (group A streptococcus), alone or in combination with Staphylococcus aureus, is an important pathogen associated with this disease.
Symptoms associated with necrotizing fasciitis include the presence of a superficial skin lesion, swelling of the affected area, and local pain followed by numbness. Patients appear acutely ill, and fever is common. Physical examination reveals skin changes that progress from an erythema to a blue-grey discoloration. Bullous changes in the skin may be present in advanced disease. During palpation of the affected area, crepitance may suggest the presence of subcutaneous gas. The hallmark of the diagnosis of vulvar necrotizing fasciitis is the presence of woody induration extending into the inner thighs.
Laboratory studies usually confirm an anemia and associated leukocytosis. Patients may be hypocalcemic from the saponification of calcium in the subcutaneous tissue. X-ray examination may suggest the presence of subcutaneous gas.
The mainstay of therapy for patients with necrotizing fasciitis is surgical debridement. After patients have been started on broad-spectrum antimicrobial therapy (Table 2) and resuscitated with fluids, immediate surgery is in order. The first order of business is to remove all necrotic tissue with its overlying skin. Infected tissue should be resected to bleeding edges. The deep fascia should be inspected. Do not tunnel beneath the skin to remove necrotic tissue. This approach makes continued care on the ward impossible. Leave an incision that can be unpacked and inspected on the ward. Pack the wound open with povidone-iodine-impregnated gauze. Consider observing the patient in a critical care setting if her condition warrants it. A planned second exploration of the wound in 24 hours may be considered to ensure that the infectious process has ceased to spread.
Antibiotics should be continued until wound induration and the systemic signs of sepsis have disappeared. The wound should appear beefy red with granulation tissue. Continued wet-to-dry dressing changes allow many wounds to heal by secondary intention. In some cases, secondary closure or skin grafting is necessary. Consultation with a plastic surgeon may be appropriate to obtain the best cosmetic result in patients with extensive vulvar or abdominal incisions, or both.
Table 2. Antibiotic regimens useful in the treatment of serious obstetric and gynecologic infections
Combination therapy
Regimen A
Clindamycin (900 mg q 8 h) or metronidazole (500 mg q 6 h)
Plus
Gentamicin* (1.5 mg/kg q 8 h) (other aminoglycoside*) or
aztreonam (2 g q 8 h)
Plus
Ampicillin (1 g q 6 h)
Regimen B
Extended-spectrum penicillin with β-lactamase inhibitor
Ticarcillin with clavulanate (3 g/200 mg q 6 h)
Ampicillin/sulbactam (2 g/1 g q 6 h)
Piperacillin with tazobactam (3 g/375 mg q 6 h)
Plus
Gentamicin* (1.5 mg/kg q 8 h) (other aminoglycoside*) or
aztreonam (2 g q 8 h)
Single-agent therapy
Carbapenem
Imipenem/cilastatin (500 mg q 6 h)
OR
Meropenem (1 gram q 8 h)
OR
Ertapenem (1 gram q 24 h)
*Amikacin may be preferred in severely ill, immunocompromised patients who have a high probability of infection with a resistant microorganism.
TOXIC SHOCK SYNDROMES
Now, more than 30 years after the highly publicized epidemic of staphylococcal toxic shock syndrome, less than 100 cases are reported annually to the Centers for Disease Control. Recently, however, two additional microorganisms, S. pyogenes (group A streptococcus) and Clostridium sordellii, have been associated with toxic shock syndromes in obstetric and gynecologic patients. These rare but commonly fatal infections challenge clinicians both diagnostically and therapeutically (Table 3).
Table 3. Clinical manifestations of toxic shock syndromes
S. aureus | S. pyogenes | C. sordellii | |
Menstrually related | + | – | – |
Not related to menses | + | + | + |
Fever | + | + | – |
Rash | + | – | – |
Hypotension | + | + | + |
Spreading edema | – | – | + |
Multiorgan dysfunction | + | + | + |
| Hemoconcentration | – | – | + |
Bacteremia | – | – | + |
(Modified with permission from the American College of Obstetricians and Gynecologists, Precis V, p 95, 1994)
Staphylococcal Toxic Shock Syndrome
The toxic shock syndrome (TSS) associated with S. aureus is characterized by fever (greater than 38.9°C (102°F)), a diffuse or palmar erythroderma (sunburn rash) progressing to subsequent peripheral desquamation, and mucous membrane hyperemia. Vomiting and diarrhea are common, and multiple organ system dysfunction with rapid progression to hypotension and shock can be seen in severe cases. Staphylococcal TSS classically occurs in young women aged 16–30 years, with a peak onset of symptoms on day 4 of menses. The disease is less common in sexually active women. It has been associated with tampon use, especially those with synthetic fibers such as carboxymethylcellulose and polyacrylate rayon that allow increased absorption of menstrual fluid.15 Once this type of tampon was taken off the market, the incidence of menstrually-related TSS plumetted.
The pathogenesis of staphylococcal TSS involves the colonization of the vagina with S. aureus and the elaboration of an exotoxin, toxic shock syndrome toxin 1 (TSST-1), unique to S. aureus. The production of toxin appears to be amplified by vaginal tampons rich in synthetic fibers. More than 75% of young adult women have a demonstrable antibody for this toxin, and more than 95% are TSST-1 antibody-positive by the fourth decade of life. This antibody is protective and explains the low incidence of the syndrome. It also explains why the disease is more likely to occur in younger women who are less likely to have the protective antibody.
Cases of nonmenstrually related staphylococcal TSS have been associated with surgical wound infections, postpartum infections, and local infections, such as mastitis, vaginitis, and pelvic inflammatory disease. Although the occurrence is rare, given the pathogenesis of the disease, TSS is a potential outcome in any clinical scenario in which S. aureus infects tissues and produces toxin.
The diagnostic workup of a patient with suspected TSS starts with a complete physical examination to investigate signs of multiorgan involvement. A pelvic examination is performed, which includes the removal of a tampon if one is present. The organ systems potentially involved and their appropriate laboratory studies are as follows:
Cardiac: electrocardiography, cardiac enzymes
Respiratory: chest x-ray, arterial blood gas
Hepatic: liver function tests
Renal: creatinine, blood urea nitrogen, urinalysis
Hematologic: complete blood count, platelets, coagulation profile
Musculoskeletal: creatinine phosphokinase
Multiple cultures to document the presence of S. aureus and to rule out the presence of the group A streptococcus should be performed. Sites to be considered for culture should include mucous membranes (e.g., conjunctivae, oropharynx, vagina), blood (although bacteremia is uncommon), focal lesions, stool, urine, and cerebrospinal fluid (if neurologic signs are present).
Treatment of this disorder involves correcting the hypotension with fluid replacement and supporting other organ functions. Calcium supplementation, blood product transfusion, mechanical ventilation, and even renal dialysis may be necessary in severely affected patients. Antimicrobial treatment with a β-lactamase-resistant penicillin (e.g., oxacillin, nafcillin) or cephalosporin (e.g., cefazolin) is also important.
Streptococcal Toxic Shock Syndrome
A similar TSS occurs as a result of infection with a toxin-producing strain of S. pyogenes (group A streptococcus). The case definition of streptococcal TSS involves the presence of hypotension (systolic BP less than 90 mmHg) and multisystem organ involvement (more than two systems).16
The pathogenesis of streptococcal TSS involves the acquisition of S. pyogenes. Mucous membranes become colonized with this microorganism; signs of a symptomatic infection usually are not manifested. Tissue invasion occurs, usually associated with bacterial M types 1 and 3, and the presence of pyrogenic exotoxins A or B, or both, leads to shock, multiorgan failure, and tissue destruction. Antibody to these exotoxins is probably protective. Streptococcal TSS has been reported in cases of septic abortion, postpartum endomyometritis, necrotizing fasciitis, and postoperative infection.
Localized pain is the most common presenting symptom occurring in 85% of cases. Influenza-like symptoms are also common. Patients typically are febrile and appear toxic, and they also may be mentally confused. There may be evidence of a localized soft-tissue infection, but the source of infection can not be determined in as many as 50% of cases.17 Most (95%) patients with streptococcal TSS have S. pyogenes bacteremia. A rash is not a prominent presenting sign in patients with streptococcal TSS.
Treatment of this serious infection involves an antibiotic with good activity against group A streptococci (e.g., penicillin) and, in many cases, prompt surgical debridement. Intravenous immunoglobulin therapy also may be helpful.18 Obviously, severely affected patients will require supportive measures as well.
Clostridium sordellii -Associated Toxic Shock Syndrome
The TSS associated with C. sordellii is characterized by the sudden onset of weakness, nausea, and vomiting followed by progressive refractory hypotension associated with local and spreading edema. It is distinguished from staphylococcal TSS by the absence of: (1) S. aureus, (2) fever, and (3) rash. C. sordellii- associated toxic shock (CATS) has been described in patients with medical abortion19and it has also been associated with episiotomy infection, postpartum infections, wound infections, a vaginal foreign body, and a degenerating cervical myoma.20 Moreover, fatal Clostridum sordellii-associated toxic shock syndrome has now been reported in six women undergoing medical abortion with mifepristone.21, 22
The pathogenesis involves the production of edema-producing C. difficile-like toxins by C. sordellii. This microorganism is fortunately only a rare inhabitant of the vagina. Antibody to the toxins is probably protective.
Treatment with broad-spectrum antibiotic therapy with activity against anaerobic bacteria such as C. sordellii should be initiated. Surgical therapy to debride necrotic soft tissue should be instituted promptly. Intravenous immunoglobulin therapy may be helpful. Despite these interventions, CATS is uniformly fatal.
Summary
All TSS patients present with an evolving clinical picture resulting in shock. Management involves clinically supporting the patient, beginning broad-spectrum antimicrobial therapy, and in selected cases, surgical intervention. Once the diagnosis is clarified, more specific antibiotic treatment, such as penicillin for streptococcal TSS or a β-lactamase-resistant antibiotic for staphylococcal TSS, can be initiated. Initial considerations during the physical examination involve the removal of tampons, sponges, or other foreign bodies from the vagina. Aerobic cultures of the mucous membranes (e.g., oropharynx, vagina), blood, focal lesions (e.g., endometrium), and urine should be performed to determine the pathogen involved. Anaerobic cultures can be recommended for the endometrium. Fluid replacement to correct hypotension, and intensive-care monitoring is required.
SEPTIC SHOCK
The majority of sepsis is observed in critically ill hospitalized patients and is due to nosocomial (hospital-acquired) microorganisms, however the obstetrician/gynecologist needs to be more aware of those previously healthy patients presenting with infections associated with pregnancy or PID or following gyn procedures. In addition, vulvar infections in patients with diabetes can become severe.
Table 4. Ob/gyn scenarios associated with sepsis
Obstetrical procedures
Abortion
Vaginal delivery
Episiotomy
Cesarean delivery
Gynecological procedures
Hysterectomy
Vulvar infection
Complicated by diabetes
Pelvic inflammatory disease
Ruptured tuboovarian abscess
Pathophysiology
Sepsis is a systemic inflammatory response (SIRS) resulting from infection. Sepsis with hypotension (systolic blood pressure <90 mmHg, or a reduction of 40 mmHg from baseline) despite adequate fluid resuscitation, in conjunction with organ dysfunction and perfusion abnormalities (e.g., lactic acidosis, oliguria, obtundation, and so forth) is referred to as septic shock.2 The prevailing theory has been that sepsis represents an uncontrolled inflammatory response. However, this finding may only represent the initial phase of SIRS which is then followed by a shift toward an anti-inflammatory immunosuppressive state.23
In a susceptible host, a particularly virulent pathogen with lipopolysaccharide (LPS) or producing a superantigen can initiate a cascade of immunologic events that can result in a severe systemic inflammatory response (SRS) and subsequent multiple organ dysfunction. LPS, a major component of the outer membrane of Gram-negative bacteria, is an endotoxin, and induces a strong immune response. Superantigens are exotoxins that exhibit highly potent mitogenic activity directed towards T lymphocytes. Compared to a normal antigen-induced T-cell response where 0.001–0.0001% of the body’s T-cells are activated, superantigens are capable of activating up to 20% of the body’s T-cells.18, While the initial response is hyperinflammatory, it rapidly progresses to hypoinflammatory (immunosuppressive). Most deaths occur during the a prolonged hypoimmune state.23
Diagnosis
Clinicians may be surprised to find that the criteria for the diagnosis of sepsis and SIRS are rather modest as noted in Table 5. Sepsis syndromes in ob/gyn patients caused by either Gram positive or Gram negative microorganisms cannot be differentiated clinically.1
Table 2. Criteria for the diagnosis of sepsis/systemic inflammatory response syndrome*
Temperature: >38°C (100.4°F) or <36°C (96.8°F)
Heart rate: >90 beats/min
Respiratory rate: >20 breaths/min or PaCO2 less than 32 mmHg
White blood cell count: >12,000 cells/µl or <4000 cells/µl or >10% immature forms
*Two or more must be present
The vital signs are a crucial part of the physical examination. Patients may be febrile or hypothermic, so the absence of fever should not dissuade the clinician from considering a diagnosis of infection. When fever is present, temperatures exceeding 38.9°C (102°F) should be especially concerning. Although tachycardia is common with fever and a pulse of greater than 90 beats/min qualifies as one of the criteria for sepsis. Hypotension with a systolic blood pressure <90 mmHg, or a reduction of 40 mmHg from baseline also suggests the diagnosis of sepsis. The respiratory rate is an often ignored component of the vital signs. Tachypnea, a respiratory rate exceeding 20 breaths/min, could signify an underlying metabolic acidosis and the clinician should consider an arterial blood gas as part of this patient’s evaluation.
The history and physical examination are commonly inconsistent in women with sepsis. This leaves laboratory testing as the lynchpin for its diagnosis. A complete blood count and basic metabolic panel are recommended. While an elevated white blood cell count >12,000 cells/µl commonly points to infection, clinicians are often inappropriately reassured by normal or low white blood cell counts. Be cautious in these cases! A normal white blood cell count and more importantly a low white blood cell count (<4,000 cells/µl) needs further interpretation of the differential. A shift to immature forms can be striking, sometimes approaching 50%, and even in the face of a normal or low white blood cell count should suggest the possibility of sepsis. Consider repeating the white blood cell count during a period of observation to determine if it is precipitously falling, another indication of a severe underlying infection.
Patients with sepsis are often dehydrated so consider the hemoglobin and hematocrit. Evidence of hemoconcentration with a hemoglobin >15 mg/dl and a hematocrit of >45 vol% is another clue. The next test to discern is the basic metabolic panel with a calculation of the anion gap. This simple calculation subtracts the negative ions from the positive ions (Na+ + K+ – Cl- + HCO3–) (normal = 8–16 mEq/ml). An elevated anion gap (>16) suggests a significant metabolic acidosis. Further evaluation can then be undertaken with an arterial blood gas (ABG). An ABG confirming acidosis (pH <7.40) and a depressed bicarbonate is cause for concern. Three other laboratory tests should be considered. An elevated lactic acid further confirms the presence of a metabolic acidosis. An elevated serum creatinine often shows evidence of renal impairment which precedes hypotension. An elevated creatinine phosphokinase (CPK) suggests deeper soft tissue infections such as necrotizing fasciitis or myositis.
Treatment24
The resuscitation of a patient in severe sepsis should begin as soon as the syndrome is recognized. During the first six hours of resuscitation, the goals should include maintaining the central venous pressure between 8 and 12 mmHg, the mean arterial pressure >65 mmHg, urine output >30 ml per hour, and a mixed venous oxygen saturation >70%. Early goal-directed therapy has been shown to improve survival for emergency department patients presenting with septic shock.
Fluid resuscitation may consist of natural or artificial colloids or crystalloids. A fluid challenge in patients with suspected hypovolemia may be given at a rate of 500–1000 ml of crystalloids or 300–500 ml of colloids over 30 minutes and repeated based on response. Although the cause of tachycardia in septic patients is multifactorial, a decrease in the elevated pulse with fluid resuscitation is often a useful marker of improving intravascular filling.
The administration of appropriate antibiotics significantly enhances survival. Before the initiation of therapy, two or three separate blood specimens should be sent for aerobic and anaerobic cultures; appropriate genital tract cultures (including urine) also should be sent. Therapy should be initiated promptly (preferably within an hour of establishing the diagnosis) and usually should include at least two bactericidal antibiotics, one being an aminoglycoside, because of the frequency of Gram-negative sepsis as the cause of shock. Particularly useful regimens are noted in Table 2. Clindamycin has excellent anaerobic coverage, gentamicin covers aerobic Gram-negative rods, and ampicillin covers most gram-positive pathogens, including the enterococci. Increased aminoglycoside loading doses may be required because of an expanded volume of distribution. The carbapenems or a β-lactam antibiotic with a β-lactamase inhibitor with an aminoglycoside can be used in this clinical scenario because there antimicrobial spectrum is similar to the traditional triple antibiotic therapy.
Some patients require surgical intervention in addition to antibiotic administration. Evaluation for a focus of infection, usually with the help of imaging studies such as computed tomography, is important. Source control measures such as drainage of an abscess or the debridement of infected necrotic tissue offer definitive control of a source of ongoing microbial contamination. Patients with septic abortion should undergo dilatation and curettage, those with necrotizing fasciitis should undergo extensive debridement, and patients with extensive uterine myonecrosis should undergo hysterectomy. If a pelvic abscess is present, drainage via laparotomy, colpotomy, or percutaneous catheter should be performed. Indicated surgery never should be delayed because the patient appears to be unstable. In point of fact, surgery may be the only means to stabilize a severely infected patient.17
REFERENCES
Lew P, Despont J, Perrin L et al: Demonstration of a local exhaustion of complement components and of an enzymatic degradation of immunoglobulins in pleural empyema: A possible factor favouring the persistence of local bacterial infections. Clin Exp Immunol 42: 506, 1980 |
|
Clayton J: Radiologic considerations in pelvic and intra-abdominal infections. In Pastorek JG III (ed): Obstetric and Gynecologic Infectious Disease. New York, Raven Press, 1994 |
|
Weinstein WM, Onderdonk AB, Bartlett JG, Gorbach SL: Experimental intraabdominal abscesses in rats: Development of an experimental model. Infect Immun 10: 1250, 1974 |
|
Sweet RL, Gibbs RS: Mixed anaerobic-aerobic pelvic infection and pelvic abscess. In Sweet RL, Gibbs RS (eds): Infectious Diseases of the Female Genital Tract, pp 75–108. Baltimore, Williams & Wilkins, 1990 |
|
Sweet RL, Gibbs RS: Postpartum infection. In Sweet RL, Gibbs RS (eds): Infectious Diseases of the Female Genital Tract, pp 356–373. Baltimore, Williams & Wilkins, 1990 |
|
Reed SD, Landers DV, Sweet RL: Antibiotic treatment of tuboovarian abscess: Comparison of broad-spectrum &b.beta;-lactam agents versus clindamycin-containing regimens. Am J Obstet Gynecol 164: 1556, 1991 |
|
Hevron JE, Llorens AS: Management of postoperative abscess following gynecologic surgery. Obstet Gynecol 47: 553, 1976 |
|
Fabiszewski NL, Sumkin JH, Johns CM: Contemporary radiologic percutaneous abscess drainage in the pelvis. Clin Obstet Gynecol. 1993 Jun;36(2):445-56. |
|
Worthen NJ, Gunning JE: Percutaneous drainage of pelvic abscesses: management of the tubo-ovarianabscess. J Ultrasound Med. 1986 Oct;5(10):551-6. |
|
Duff P, Gibbs RS: Pelvic vein thrombophlebitis: Diagnostic dilemma and therapeutic challenge. Obstet Gynecol Surv 38: 365, 1983 |
|
Dunn LJ, Van Voorhis LW. Enigmatic fever and pelvic thrombophlebitis. N Engl J Med 1967;276:265-8 |
|
Brown CE, Stettler RW, Twickler D, Cunningham FG. Puerperal septic pelvic thrombophlebitis: Incidence and response to heparin therapy. Am J Obstet Gynecol 1999:181:143-8 |
|
Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: Diagnosis and management. Clin Infect Dis 2007;44:705-10 |
|
Soper DE: Necrotizing fasciitis. In Pastorek JG III (ed): Obstetric and Gynecologic Infectious Disease, pp 157–163. New York, Raven Press, 1994 |
|
Wager GP: Toxic shock syndrome: A review. Am J Obstet Gynecol 146: 93, 1983 |
|
Working Group on Severe Streptococcal Infections: Defining the group A streptococcal toxic shock syndrome: Rational and consensus definition. JAMA 269:390, 1993 |
|
Stevens DL. Group A streptococcal sepsis. Current Infectious Disease Reports 2003;5:379-86 |
|
Barry W, Hudgins L, Donta ST, Pesanti EL: Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA 267: 3315, 1992 |
|
Aldape MJ, Bryant AE, Stevens DL. Clostridum sordellii infection: Epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clinical Infectious Diseases 2006;43:1436-46 |
|
McGregor JA, Soper DE, Lovell G, Todd JK: Maternal deaths associated with Clostridium sordellii infection. Am J Obstet Gynecol 146: 93, 1989 |
|
Fischer M, Bhatnagar J, Guarner J, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med 2005;353(22):2352-60 |
|
Cohen AL, Bhatnagar J, Reagan S, et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet Gynecol 2007;110(5):1027-33 |
|
Hotchkiss RS, Karl KE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138-50 |
|
Dellinger RP, Carlet JM, Masur H et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septicshock. Intensive Care Med. 2004 Apr;30(4):536-55. Epub 2004 Mar 3. |